
Reduction of ketones with chiral hydride donors
When the reducing agent itself is chiral, high selectivity can be achieved even if the substrate is not chiral. Reduction of unsymmetrical ketones (prochiral ketone) is an example. A number of reagents have been used of which one of the best is
α -3-pinanyl-9-borabicyclo[3.3.1]nonane which is readily prepared from (+)-α-pinene and 9-borabicyclononane. 9-BBN itself can be prepared by reaction of borane with 1,5-cyclooctadiene. 1-octyn-3-one gives (R)-1-octyn-3-ol with 92 % optical purity.
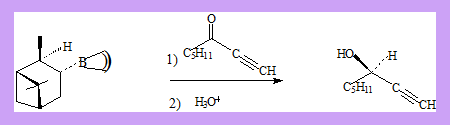
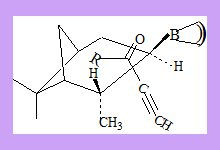
The reaction is supposed to have the transition state as shown in which the pentyl group is above the pinene ring thus preventing the approach of the hydride from one direction.
Michael addition in the presence of chiral amines leads to enantioselectivity. α-β-unsaturated amides derived from ephedrine, on Grignard addition and subsequent hydrolysis result in 3,3-disubstituted propionic acids in 79-99%(ee).
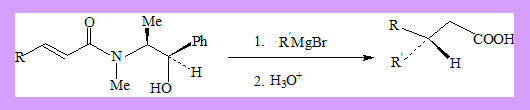
Use of chiral phase transfer catalysts should in principle give rise to enantioselectivivity. A good example is as follows.
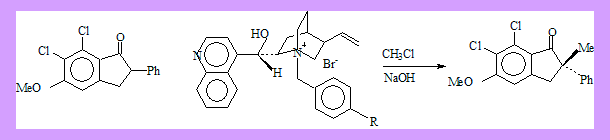
The quinquilidine ring lies behind the plane of the indanone enolate permitting Π-interaction between the benzyl group of the catalyst and the 2-phenyl group, at the same time 9-OH provides hydrogen bonding interaction so that back side approach of the alkylating agent (MeCl) is effectively prohibited, and S-(+)-2-methylindanone is formed.